Metabolite Testing Kit
Determine your patient’s
plasma PAA:PAGN ratio and
urinary PAGN level.
Look over the patient treatment profile and important biomarkers, and order a metabolite testing kit.
When plasma ammonia is elevated, increase the RAVICTI dosage to reduce the fasting ammonia level to less than half the upper limit of normal (ULN) in patients 6 years of age and older, or reduce the first ammonia level of the morning to below the ULN in infants and young children (generally less than 6 years of age).1,b
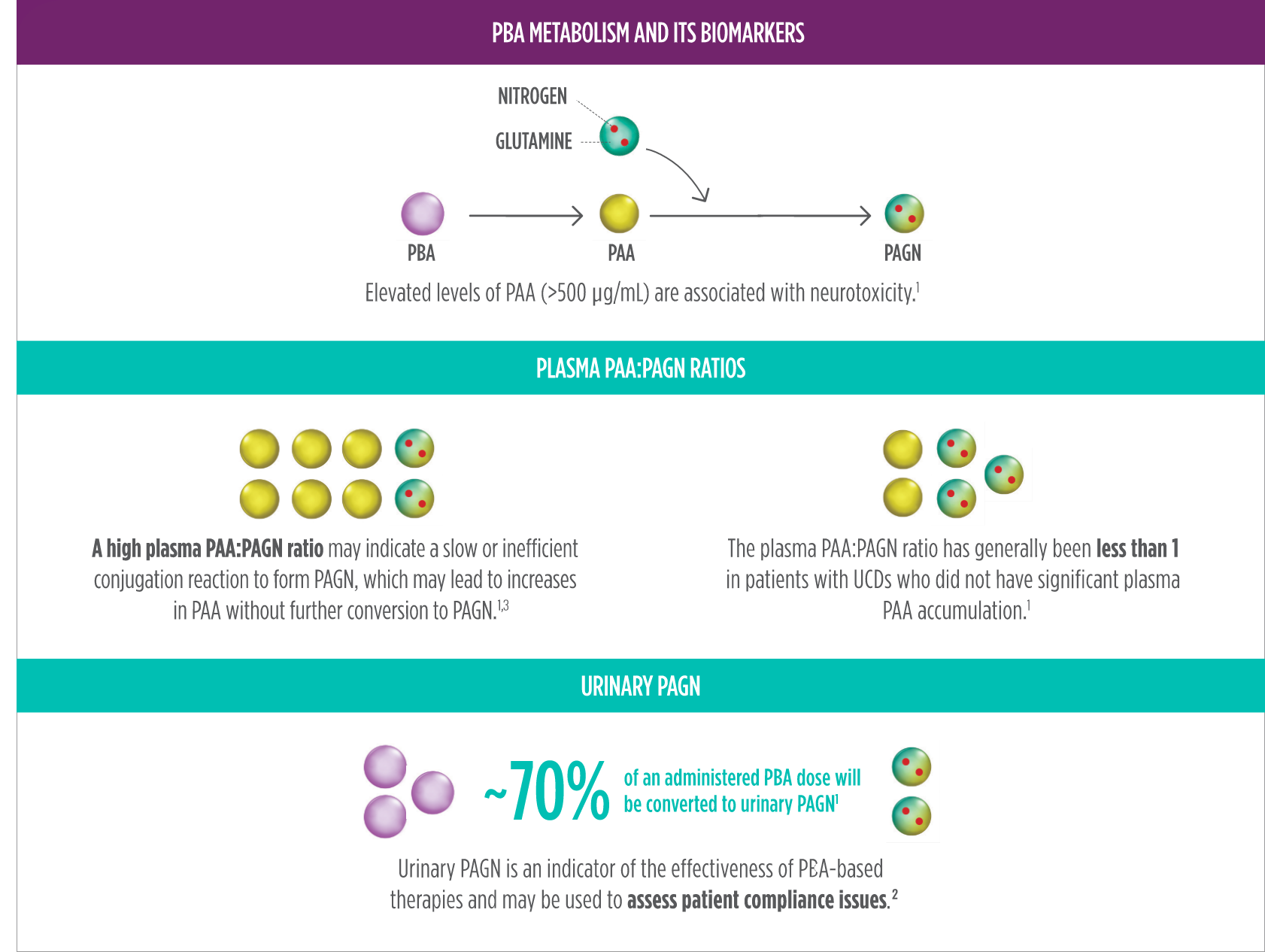
PAA is the major metabolite of RAVICTI and may be associated with neurotoxicity. The PAA:PAGN ratio has been shown to be less than 1 in patients without significant PAA accumulation. A high PAA:PAGN ratio may indicate a slow or inefficient conjugation reaction to form PAGN, which may lead to increases in PAA without further conversion to PAGN.1,2,c
If U-PAGN excretion is insufficient to cover daily dietary protein intake and the fasting ammonia is greater than half the ULN, the RAVICTI dosage should be increased.1
Periodic reassessment of BSA should be performed to ensure accurate dosing, particularly in pediatric patients who will experience significant changes in this parameter over time.1
Be sure to consider any changes in hepatic capacity. For patients with moderate to severe hepatic impairment, the recommended starting dosage is at the lower end of the recommended dosing range, and the dosage should be kept at the lowest necessary to control the patient's plasma ammonia.1
Changes in a patient’s protein intake—either prescribed or through nonadherence to diet—may require altering the dosage to compensate for greater amounts of nitrogen that need to be excreted as waste.1
bInstruct patients to abstain from eating or drinking any foods or liquids before plasma ammonia levels are to be measured.1
cConsider a patient’s use of concomitant medications such as probenecid, when making dosing adjustment decisions based on U-PAGN and PAA. Probenecid may result in decrease of the urinary excretion of PAGN.1
Determine your patient’s
plasma PAA:PAGN ratio and
urinary PAGN level.
This assessment tool can help evaluate
treatment of your patient and ensure
management of their UCD.
A representative can answer your
questions about modifying the
RAVICTI dosage.
References: 1. RAVICTI (glycerol phenylbutyrate) Oral Liquid [prescribing information] Horizon. 2. Mokhtarani M, Diaz GA, Rhead W, et al. Elevated phenylacetic acid levels do not correlate with adverse reactions in patients with urea cycle disorders or hepatic encephalopathy and can be predicted based on the plasma PAA to PAGN ratio. Mol Genet Metab. 2013;110(4):446-453. 3. Mokhtarani M, Diaz GA, Rhead W, et al. Urinary phenylacetylglutamine as dosing biomarker for patients with urea cycle disorders. Mol Genet Metab. 2012;107(3):308-314.
RAVICTI (glycerol phenylbutyrate) Oral Liquid is indicated for use as a nitrogen-binding agent for chronic management of patients with urea cycle disorders (UCDs) who cannot be managed by dietary protein restriction and/or supplementation alone. RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g. essential amino acids, arginine, citrulline, protein-free calorie supplements).
The most common adverse reactions reported in clinical trials (at least 10% of patients) were:
Please see Full Prescribing Information.
RAVICTI (glycerol phenylbutyrate) Oral Liquid is indicated for use as a nitrogen-binding agent for chronic management of patients with urea cycle disorders (UCDs) who cannot be managed by dietary protein restriction and/or supplementation alone. RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g. essential amino acids, arginine, citrulline, protein-free calorie supplements).
The most common adverse reactions reported in clinical trials (at least 10% of patients) were:
Please see Full Prescribing Information.