Welcome to the RAVICTI® (glycerol phenylbutyrate) Oral Liquid instructional dosing video. In this video you will see step-by-step instructions for administration of RAVICTI if your child has a nasogastric feeding tube.
Use for RAVICTI® (glycerol phenylbutyrate) Oral Liquid
RAVICTI is a prescription medicine used for long-term management of high blood levels of ammonia (hyperammonemia) caused by a condition called a urea cycle disorder (UCD). RAVICTI should be used if the UCD cannot be managed with a low-protein diet and dietary supplements alone. RAVICTI must be used along with a low-protein diet and in some cases, dietary supplements.
RAVICTI is not used to treat extremely high levels of ammonia in the blood (hyperammonemic crisis) in people with UCDs.
It is not known if RAVICTI is safe and effective for the treatment of N-acetylglutamate synthase (NAGS) deficiency.
Please listen to the RAVICTI Important Safety Information at the end of this video. For additional important safety information, see the Medication Guide at RAVICTI.com and discuss with your doctor.
This video is intended to complement the instructions you have already received from your doctor. Always give RAVICTI exactly as directed by your doctor.
Do not mix RAVICTI with formula or any other liquids.
Give RAVICTI just prior to feeding your child.
If your child has a feeding tube and can swallow, give RAVICTI orally.
Now, let’s get started.
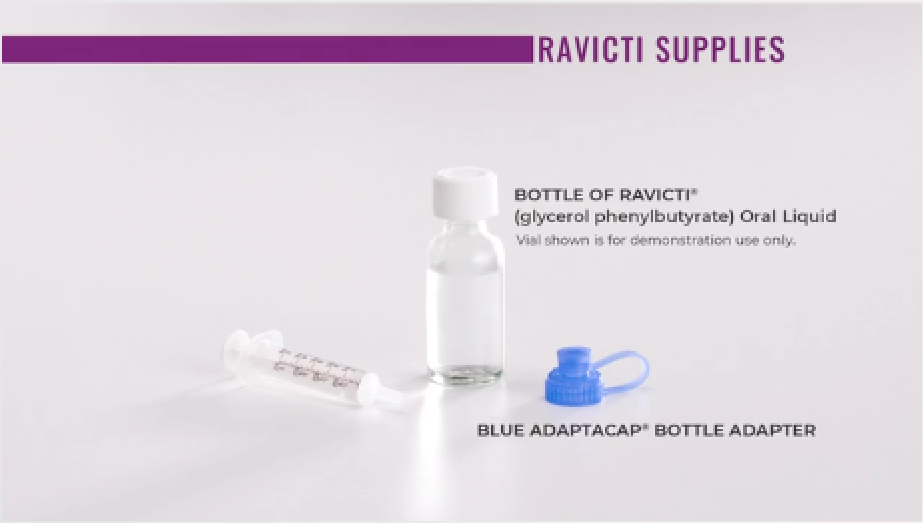
Your RAVICTI shipment includes a 25-mL bottle of RAVICTI, a blue AdaptaCap®Bottle Adapter, and an oral dosing syringe.
When dosing RAVICTI, be sure to use the oral dosing syringe provided with the product.
Have a second, larger syringe ready for flushing the feeding tube.
For the first step, remove the childproof cap on the RAVICTI bottle. To do that, push down on the cap while twisting it to the left.
Then, attach the blue AdaptaCap® Bottle Adapter onto the bottle. Put it on top of the bottle and twist it to the right.
Make sure it is secured tightly on the bottle.
While holding the RAVICTI bottle securely, place the tip of the oral dosing syringe into the AdaptaCap® Bottle Adapter.
Turn the bottle upside down with the oral dosing syringe still inserted.
Now, you’re ready to fill the oral dosing syringe with RAVICTI.
Get the medicine into the oral dosing syringe by pulling the plunger back slowly. Pulling slowly will help avoid large air bubbles from forming in the medicine.
Pull the plunger back until the stopper is even with the dosing mark. The dosing marks are on the barrel of the oral dosing syringe. Match the dosing mark with the amount of medicine prescribed by your doctor.
Then, turn the bottle upright again.
If there are large air bubbles, push the medicine back into the bottle. Do this slowly.
Then, draw up the medicine again.
Once the dose is ready, close the tab on the AdaptaCap® Bottle Adapter. Make sure it’s closed tightly.
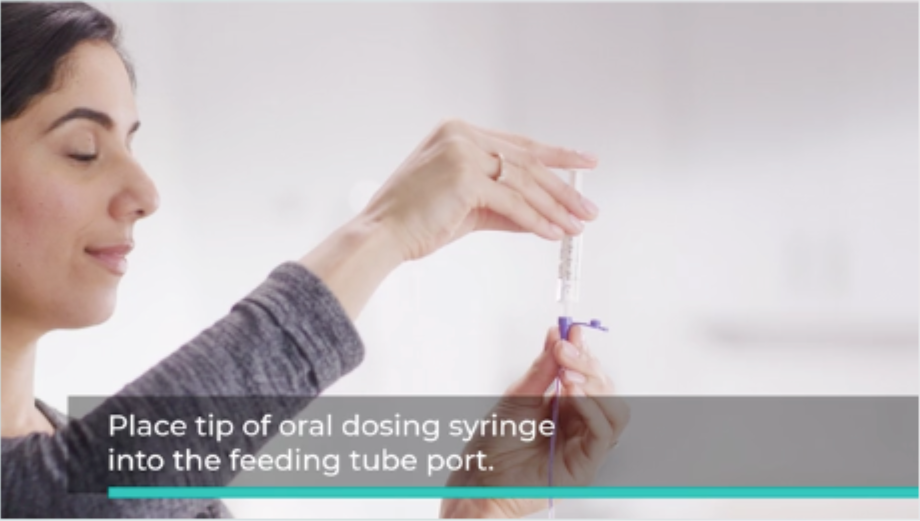
Once you have the oral dosing syringe prepared, place its tip into the feeding tube port.
Using the plunger of the oral dosing syringe, slowly and carefully push RAVICTI into the feeding tube. Make sure all of the medicine is pushed out of the oral dosing syringe.
After you have pushed all of the RAVICTI into the feeding tube, remove the oral dosing syringe and set it aside.
Because RAVICTI has a thick consistency, you will need to flush the feeding tube with water or formula to help push all of the RAVICTI through the tube.
Use the separate, larger syringe for flushing and make sure all of the water or formula drains through the feeding tube.
If your child requires less than 1 mL of RAVICTI per dose, the amount delivered through the feeding tube may be less than intended. Work with the doctor to closely monitor your child’s ammonia levels.
RAVICTI should be given multiple times throughout the day, based on the dosing schedule prescribed by your doctor. Follow these instructions and tips for giving multiple daily doses.
Give all RAVICTI doses with feedings, 3 or more times per day.
If your child is breastfed, give RAVICTI just prior to breastfeeding
You can use 1 oral dosing syringe and 1 blue AdaptaCap® Bottle Adapter for all doses each day.
You can also choose to use a new oral dosing syringe with each dose.
Do not rinse the AdaptaCap® Bottle Adapter or oral dosing syringe between doses.
After the last dose of the day, dispose of the oral dosing syringe.
If you give too much RAVICTI, call your doctor or go to the nearest hospital emergency room right away. You should consult your child’s doctor if they have missed a dose of RAVICTI.
Do not mix RAVICTI with formula or any other liquids.
If your child requires less than 1 mL of RAVICTI per dose, the amount delivered through the feeding tube may be less than intended. Work with your child’s doctor to closely monitor their ammonia levels.